Organic Chemistry Some Basic Principles And Techniques Part-3
Assessment Worksheets
This assessment will be based on: Organic Chemistry Some Basic Principles And Techniques Part-3
Study Notes and Summary
12.9 QUALITATIVE ANALYSIS OF ORGANIC COMPOUNDS
The elements present in organic compounds are carbon and hydrogen. In addition to these, they may also contain oxygen, nitrogen, sulphur, halogens and phosphorus.
12.9.1 Detection of Carbon and Hydrogen
Carbon and hydrogen are detected by heating the compound with copper(II) oxide. Carbon present in the compound is oxidised to carbon dioxide (tested with lime-water, which develops turbidity) and hydrogen to water (tested with anhydrous copper sulphate, which turns blue).
$\mathrm{C}+ \mathrm{2CuO} \xrightarrow{\Delta} 2\mathrm{Cu}+ \mathrm{CO_2}$
$2\mathrm{H}+ \mathrm{CuO} \xrightarrow{\Delta} \mathrm{Cu}+ \mathrm{H_2O}$
$\mathrm{CO_2}+ \mathrm{Ca(OH)_2} \rightarrow \mathrm{CaCO_3}\downarrow+ \mathrm{H_2O}$
$5\mathrm{H_2O}+ \underset{\text{White}}{\mathrm{CuSO_4}} \rightarrow \underset{\text{Blue}}{\mathrm{CuSO_4.5H_2O}}$
12.9.2 Detection of Other Elements
Nitrogen, sulphur, halogens and phosphorus present in an organic compound are detected by “Lassaigne’s test”. The elements present in the compound are converted from covalent form into the ionic form by fusing the compound with sodium metal. Following reactions take place:
$$
\begin{array}{lll}
\mathrm{Na}+\mathrm{C}+\mathrm{N} & \xrightarrow{\Delta} & \mathrm{NaCN} \\
2 \mathrm{Na}+\mathrm{S} & \xrightarrow{\Delta} & \mathrm{Na_2} \mathrm{~S} \\
\mathrm{Na}+\mathrm{X} & \xrightarrow{\Delta} & \mathrm{Na} \mathrm{X} \\
& & (\mathrm{X}=\mathrm{Cl}, \text { Br or } \mathrm{I})
\end{array}
$$
C, N, S and X come from organic compound.
Cyanide, sulphide and halide of sodium so formed on sodium fusion are extracted from the fused mass by boiling it with distilled water. This extract is known as sodium fusion extract.
(A) Test for Nitrogen
The sodium fusion extract is boiled with iron(II) sulphate and then acidified with
concentrated sulphuric acid. The formation of Prussian blue colour confirms the presence of nitrogen. Sodium cyanide first reacts with iron(II) sulphate and forms sodium hexacyanidoferrate(II). On heating with concentrated sulphuric acid some iron(II) ions are oxidised to iron(III) ions which react with sodium hexacyanidoferrate(II) to produce iron(III) hexacyanidoferrate(II) (ferriferrocyanide) which is Prussian blue in colour.
$$
\begin{aligned}
& 6 \mathrm{CN}^{-}+\mathrm{Fe}^{2+} \rightarrow\left[\mathrm{Fe}(\mathrm{CN}) _{6}\right]^{4-} \\
& 3\left[\mathrm{Fe}(\mathrm{CN}) _{6}\right]^{4-}+4 \mathrm{Fe}^{3+} \xrightarrow {xH_2O} \mathrm{Fe} _{4}\left[\mathrm{Fe}(\mathrm{CN}) _{6}\right] _{3} . xH_2O\\
& \hspace{40 mm} \text { Prussian Blue }
\end{aligned}
$$
(B) Test for Sulphur
(a) The sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate of lead sulphide indicates the presence of sulphur.
$$
\begin{aligned}
& \mathrm{S}^{2-}+\mathrm{Pb}^{2+} \longrightarrow \underset{\text{Black}}{\mathrm{PbS}}
\end{aligned}
$$
(b) On treating sodium fusion extract with sodium nitroprusside, appearance of a violet colour further indicates the presence of sulphur.
$$
\mathrm{S}^{2-}+\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NO}\right]^{2-} \longrightarrow \quad \underset{\text { Violet }}{\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NOS}\right]^{4-}}
$$
In case, nitrogen and sulphur both are present in an organic compound, sodium thiocyanate is formed. It gives blood red colour and no Prussian blue since there are no free cyanide ions.
$$
\begin{aligned}
& \mathrm{Na}+\mathrm{C}+\mathrm{N}+\mathrm{S} \rightarrow \mathrm{NaSCN} \\
& \mathrm{Fe}^{3+}+3 \mathrm{SCN}^{-} \rightarrow \mathrm{Fe}(\mathrm{SCN})^{2+}
\end{aligned}
$$
Blood Red
If sodium fusion is carried out with excess of sodium, the thiocyanate decomposes to yield cyanide and sulphide. These ions give their usual tests.
$$
\mathrm{NaSCN}+2 \mathrm{Na} \longrightarrow \mathrm{NaCN}+\mathrm{Na_2} \mathrm{~S}
$$
(C) Test for Halogens
The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. A white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish precipitate, sparingly soluble in ammonium hydroxide shows the presence of bromine and a yellow precipitate, insoluble in ammonium hydroxide shows the presence of iodine.
$$
\begin{aligned}
& \mathrm{X}^{-}+\mathrm{Ag}^{+} \rightarrow \mathrm{AgX} \\
& {[\mathrm{X} \quad \text {represents a halogen} \quad \mathrm{Cl}, \mathrm{Br} \text { or } \mathrm{I}]}
\end{aligned}
$$
If nitrogen or sulphur is also present in the compound, the sodium fusion extract is first boiled with concentrated nitric acid to decompose cyanide or sulphide of sodium formed during Lassaigne’s test. These ions would otherwise interfere with silver nitrate test for halogens.
(D) Test for Phosphorus
The compound is heated with an oxidising agent (sodium peroxide). The phosphorus present in the compound is oxidised to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.
$
\begin{aligned}
& \mathrm{Na} _{3} \mathrm{PO} _{4}+3 \mathrm{HNO} _{3} \rightarrow \mathrm{H} _{3} \mathrm{PO} _{4}+3 \mathrm{NaNO} _{3} \\
& \mathrm{H} _{3} \mathrm{PO} _{4}+\underset{\text{Ammonium molybdate}}{12\left(\mathrm{NH} _{4}\right) _{2} \mathrm{MoO} _{4}}+2 \mathrm{HNO} _{3} \rightarrow \underset{\text{ Ammonium phosphomolybdate }}{\left(\mathrm{NH} _{4}\right) _{3} \cdot \mathrm{PO} _{4} \cdot 12 \mathrm{MoO} _{3}}+21 \mathrm{NH} _{4} \mathrm{NO} _{3}+12 \mathrm{H} _{2} \mathrm{O}
\end{aligned}
$
12.10 QUANTITATIVE ANALYSIS
Quantitative analysis of compounds is very important in organic chemistry. It helps chemists in the determination of mass per cent of elements present in a compound. You have learnt in Unit-1 that mass per cent of elements is required for the determination of emperical and molecular formula.
The percentage composition of elements present in an organic compound is determined by the following methods:
12.10.1 Carbon and Hydrogen
Both carbon and hydrogen are estimated in one experiment. A known mass of an organic compound is burnt in the presence of excess of oxygen and copper(II) oxide. Carbon and hydrogen in the compound are oxidised to carbon dioxide and water respectively.
$\mathrm{C_\mathrm{x}} \mathrm{H_\mathrm{y}}+(\mathrm{x}+\mathrm{y} / 4)_2 \mathrm{O_2} \longrightarrow \mathrm{xCO_2}+(\mathrm{y} / 2) \mathrm{H_2O}$
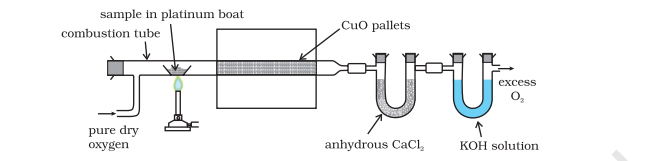
Fig.8.14 Estimation of carbon and hydrogen. Water and carbon dioxide formed on oxidation of substance are absorbed in anhydrous calcium chloride and potassium hydroxide solutions respectively contained in U tubes.
The mass of water produced is determined by passing the mixture through a weighed U-tube containing anhydrous calcium chloride. Carbon dioxide is absorbed in another U-tube containing concentrated solution of potassium hydroxide. These tubes are connected in series (Fig. 12.14). The increase in masses of calcium chloride and potassium hydroxide gives the amounts of water and carbon dioxide from which the percentages of carbon and hydrogen are calculated.
Let the mass of organic compound be $\mathrm{m} g$, mass of water and carbon dioxide produced be $m_{1}$ and $m_{2}$ g respectively;
Percentage of carbon $=\dfrac{12 \times \mathrm{m_2} \times 100}{44 \times \mathrm{m}}$
Percentage of hydrogen $=\dfrac{2 \times m_{1} \times 100}{18 \times m}$
Problem 12.20
On complete combustion, $0.246 \mathrm{~g}$ of an organic compound gave $0.198 \mathrm{~g}$ of carbon dioxide and $0.1014 \mathrm{~g}$ of water. Determine the percentage composition of carbon and hydrogen in the compound.
Solution
Percentageof carbon $=\dfrac{12 \times 0.198 \times 100}{44 \times 0.246}$
$
=21.95 \%
$
Percentage of hydrogen $=\dfrac{2 \times 0.1014 \times 100}{18 \times 0.246}$
$
=4.58 \%
$
12.10.2 Nitrogen
There are two methods for estimation of nitrogen: (i) Dumas method and (ii) Kjeldahl’s method.
(i) Dumas method: The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water.
$$
\begin{aligned}
& \mathrm{C} _{x} \mathrm{H} _{y} \mathrm{~N} _{z}+[2 x+y / 2] \mathrm{CuO} \rightarrow x \mathrm{CO} _{2}+y / 2 \mathrm{H} _{2} \mathrm{O}+z / 2 \mathrm{~N} _{2}+(2 x+y / 2) \mathrm{Cu}
\end{aligned}
$$
Traces of nitrogen oxides formed, if any, are reduced to nitrogen by passing the gaseous mixture over a heated copper gauze. The mixture of gases so produced is collected over an aqueous solution of potassium hydroxide which absorbs carbon dioxide. Nitrogen is collected in the upper part of the graduated tube (Fig.8.15).
Let the mass of organic compound $=\mathrm{mg}$
Volume of nitrogen collected $=V_{1} \mathrm{~mL}$
Room temperature $=T_{1} \mathrm{~K}$
Volume of nitrogen at STP $=\dfrac{P_{1} V_{1} \times 273}{760 \times T_{1}}$
(Let it be $\mathrm{V} \mathrm{mL}$ )
Where $p_{1}$ and $V_{1}$ are the pressure and volume of nitrogen, $p_{1}$ is different from the atmospheric pressure at which nitrogen gas is collected. The value of $p_{1}$ is obtained by the relation;
$p_{1}=$ Atmospheric pressure – Aqueous tension $22400 \mathrm{~mL} \mathrm{~N_2}$ at STP weighs $28 \mathrm{~g}$.
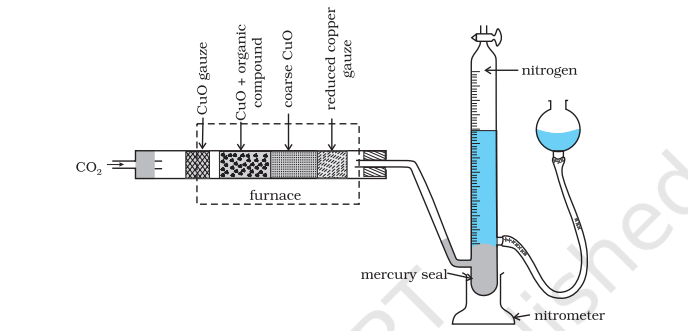
Fig. 12.15 Dumas method. The organic compound yields nitrogen gas on heating it with
copper(II) oxide in the presence of CO2 gas. The mixture of gases is collected over
potassium hydroxide solution in which CO2 is absorbed and volume of nitrogen
gas is determined.
$\mathrm{V} \quad \mathrm{mL} \quad \mathrm{N}{ _2}$ at STP weighs $=\dfrac{28 \times \mathrm{V}}{22400} \mathrm{~g}$
Percentage of nitrogen $=\dfrac{28 \times \mathrm{V} \times 100}{22400 \times \mathrm{m}}$
Problem 12.21
In Dumas’ method for estimation of nitrogen, $0.3 \mathrm{~g}$ of an organic compound gave $50 \mathrm{~mL}$ of nitrogen collected at $300 \mathrm{~K}$ temperature and $715 \mathrm{~mm}$ pressure. Calculate the percentage composition of nitrogen in the compound. (Aqueous tension at $300 \mathrm{~K}=15 \mathrm{~mm}$ )
Solution
Volume of nitrogen collected at $300 \mathrm{~K}$ and $715 \mathrm{~mm}$ pressure is $50 \mathrm{~mL}$
Actual pressure $=715-15=700 \mathrm{~mm}$
Volume of nitrogen at STP $\dfrac{273 \times 700 \times 50}{300 \times 760}$ $41.9 \mathrm{~mL}$
$22,400 \mathrm{~mL}$ of $\mathrm{N_2}$ at STP weighs $=28 \mathrm{~g}$
$
\begin{aligned}
& 41.9 \mathrm{~mL} \text { of nitrogen weighs }=\dfrac{28 \times 41.9}{22400} \mathrm{~g} \\
& \begin{aligned}
\text { Percentage of nitrogen } & =\dfrac{28 \times 41.9 \times 100}{22400 \times 0.3} \\
& =17.46 \%
\end{aligned}
\end{aligned}
$
(ii) Kjeldahl’s method: The compound containing nitrogen is heated with concentrated sulphuric acid. Nitrogen in the compound gets converted to ammonium sulphate (Fig. 12.16). The resulting acid mixture is then heated with excess of sodium hydroxide. The liberated ammonia gas is absorbed in an excess of standard solution of sulphuric acid. The amount of ammonia produced is determined by estimating the amount of sulphuric acid consumed in the reaction. It is done by estimating unreacted sulphuric acid left after the absorption of ammonia by titrating it with standard alkali solution. The difference between the initial amount of acid taken and that left after the reaction gives the amount of acid reacted with ammonia.
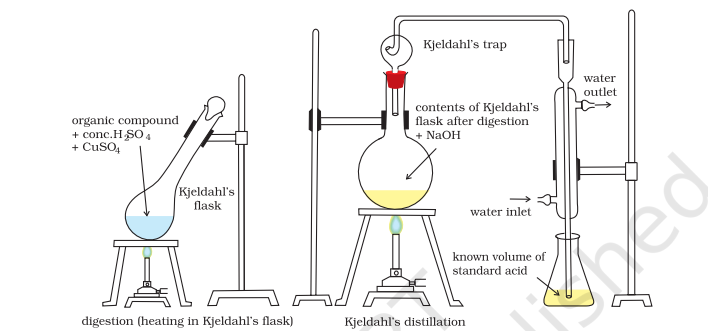
Fig.8.16 Kjeldahl method. Nitrogen-containing compound is treated with concentrated H2SO4 to get ammonium sulphate which liberates ammonia on treating with NaOH; ammonia is absorbed
in known volume of standard acid.
Organic compound $+\mathrm{H_2} \mathrm{SO_4} \longrightarrow\left(\mathrm{NH_4}\right)_{2} \mathrm{SO_4}$
$
\begin{aligned}
& \xrightarrow{2 \mathrm{NaOH}} \mathrm{Na_2} \mathrm{SO_4}+2 \mathrm{NH_3}+2 \mathrm{H_2} \mathrm{O} \\
& 2 \mathrm{NH_3}+\mathrm{H_2} \mathrm{SO_4} \longrightarrow\left(\mathrm{NH_4}\right)_{2} \mathrm{SO_4}
\end{aligned}
$
Let the mass of organic compound taken $=\mathrm{mg}$
Volume of $\mathrm{H_2} \mathrm{SO_4}$ of molarity, M, taken $=V \mathrm{~mL}$
Volume of $\mathrm{NaOH}$ of molarity, M, used for titration of excess of $\mathrm{H_2} \mathrm{SO_4}=V_{1} \mathrm{~mL}$
$V_{1} \mathrm{~mL}$ of $\mathrm{NaOH}$ of molarity $\mathrm{M}$
$
=V_{1} / 2 \mathrm{~mL} \text { of } \mathrm{H_2} \mathrm{SO_4} \text { of molarity } \mathrm{M}
$
$\left(V-V_{1} / 2\right) \mathrm{mL}$ of $\mathrm{H_2} \mathrm{SO_4}$ of molarity $\mathrm{M}$
$ = 2\left(V-V_{1} / 2\right) \mathrm{mL}$ of $\mathrm{NH_3} \text{ solution of molarity M } $
$1000 \mathrm{~mL}$ of $1 \mathrm{M} \mathrm{NH_3}$ solution contains $17 \mathrm{~g} \mathrm{NH_3}$ or $14 \mathrm{~g}$ of $\mathrm{N}$
$2\left(V-V_{1} / 2\right) \mathrm{mL}$ of $\mathrm{NH_3}$ solution of molarity M contains:
$
\dfrac{14 \times \mathrm{M} \times 2\left(\mathrm{~V}-\mathrm{V_1} / 2\right)}{1000} \mathrm{gN}
$
Percentage of $\mathrm{N}=\dfrac{14 \times \mathrm{M} \times 2\left(\mathrm{~V}-\mathrm{V_1} / 2\right)}{1000} \times \dfrac{100}{\mathrm{~m}}$
$
=\dfrac{1.4 \times \mathrm{M} \times 2(\mathrm{~V}-\mathrm{V} / 2)}{\mathrm{m}}
$
Kjeldahl method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring (e.g. pyridine) as nitrogen of these compounds does not change to ammonium sulphate under these conditions.
Problem 12.22
During estimation of nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from $0.5 \mathrm{~g}$ of the compound in Kjeldahl’s estimation of nitrogen, neutralized $10 \mathrm{~mL}$ of $1 \mathrm{M} \mathrm{H_2} \mathrm{SO_4}$. Find out the percentage of nitrogen in the compound.
Solution
$1 \mathrm{M}$ of $10 \mathrm{~mL} \mathrm{H_2} \mathrm{SO_4}=1 \mathrm{M}$ of $20 \mathrm{~mL} \mathrm{NH_3}$ $1000 \mathrm{~mL}$ of $1 \mathrm{M}$ ammonia contains $14 \mathrm{~g}$ nitrogen
$20 \mathrm{~mL}$ of $1 \mathrm{M}$ ammonia contains
$
\dfrac{14 \times 20}{1000} \mathrm{~g} \text { nitrogen }
$
Percentage of nitrogen $=\dfrac{14 \times 20 \times 100}{1000 \times 0.5}=56.0 \%$
12.10.3 Halogens
Carius method: A known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as Carius tube, (Fig.12.17)
in a furnace. Carbon and hydrogen present in the compound are oxidised to carbon dioxide and water. The halogen present forms the corresponding silver halide $(\operatorname{AgX})$ . It is filtered, washed, dried and weighed.

Fig. 12.17 Carius method. Halogen containing organic compound is heated with fuming nitric acid in the presence of silver nitrate.
Let the mass of organic compound taken $=\mathrm{m} \mathrm{g}$
Mass of $\mathrm{AgX}$ formed $=\mathrm{m_1} \mathrm{~g}$ $1 \mathrm{~mol}$ of $\mathrm{AgX}$ contains $1 \mathrm{~mol}$ of $\mathrm{X}$
Mass of halogen in $\mathrm{m_1} \mathrm{~g}$ of $\mathrm{AgX}$
$
=\dfrac{\text { atomic mass of } \mathrm{X} \times \mathrm{m_1} \mathrm{~g}}{\text { molecular mass of } \mathrm{AgX}}
$
Percentage of halogen
$
=\dfrac{\text { atomic mass of } X \times \mathrm{m_1} g}{\text { molecular mass of } \mathrm{AgX}}
$
Problem 12.23
In Carius method of estimation of halogen, $0.15 \mathrm{~g}$ of an organic compound gave $0.12 \mathrm{~g}$ of $\mathrm{AgBr}$. Find out the percentage of bromine in the compound.
Solution
Molar mass of $\mathrm{AgBr}=108+80$
$
=188 \mathrm{~g} \mathrm{~mol}^{-1}
$
$188 \mathrm{~g}$ AgBr contains $80 \mathrm{~g}$ bromine
$0.12 \mathrm{~g}$ AgBr contains $\dfrac{80 \times 0.12}{188} \mathrm{~g}$ bromine
$
\begin{aligned}
\text { Percentage of bromine } & =\dfrac{80 \times 0.12 \times 100}{188 \times 0.15} \\
& =34.04 \%
\end{aligned}
$
12.10.4 Sulphur
A known mass of an organic compound is heated in a Carius tube with sodium peroxide or fuming nitric acid. Sulphur present in the compound is oxidised to sulphuric acid. It is precipitated as barium sulphate by adding excess of barium chloride solution in water. The precipitate is filtered, washed, dried and weighed. The percentage of sulphur can be calculated from the mass of barium sulphate.
Let the mass of organic
$
\text { compound taken }=\mathrm{mg}
$
and the mass of barium
$
\text { sulphate formed }=\mathrm{m_1} \mathrm{~g}
$
$1 \mathrm{~mol} \text{ of } \mathrm{BaSO_4}=233 \mathrm{~g} \mathrm{BaSO_4}=32 \mathrm{~g}$ sulphur
$\mathrm{m_1} \mathrm{~g} \mathrm{BaSO_4}$ contains $\dfrac{32 \times m_{1}}{233}$ g sulphur
$
\text { Percentage of sulphur }=\dfrac{32 \times m_{1} \times 100}{233 \times m}
$
Problem 12.24
In sulphur estimation, $0.157 \mathrm{~g}$ of an organic compound gave $0.4813 \mathrm{~g}$ of
barium sulphate. What is the percentage of sulphur in the compound?
Solution
Molecular mass of $\mathrm{BaSO_4}=137+32+64$
$
=233 \mathrm{~g}
$
$233 \mathrm{~g} \mathrm{BaSO_4}$ contains $32 \mathrm{~g}$ sulphur
$0.4813 \mathrm{~g} \mathrm{BaSO_4}$ contains $\dfrac{32 \times 0.4813}{233} \mathrm{~g}$
$\mathrm{~g}$ sulphur
Percentage of sulphur $=\dfrac{32 \times 0.4813 \times 100}{233 \times 0.157}$
$
=42.10 \%
$
12.10.5 Phosphorus
A known mass of an organic compound is heated with fuming nitric acid whereupon phosphorus present in the compound is oxidised to phosphoric acid. It is precipitated as ammonium phosphomolybdate, $\left(\mathrm{NH _4}\right) _{3}$ $\mathrm{PO _4} \cdot 12 \mathrm{MoO _3}$, by adding ammonia and ammonium molybdate. Alternatively, phosphoric acid may be precipitated as $\mathrm{MgNH _4} \mathrm{PO _4}$ by adding magnesia mixture which on ignition yields $\mathrm{Mg _2} \mathrm{P _2} \mathrm{O _7}$.
Let the mass of organic compound taken $=\mathrm{mg}$ and mass of ammonium phospho molydate $=\mathrm{m_1} \mathrm{~g}$
Molar mass of $\left(\mathrm{NH _4}\right) _{3} \mathrm{PO _4} \cdot 12 \mathrm{MoO _3}=1877 \mathrm{~g}$ Percentage of phosphorus $=\dfrac{31 \times m _{1} \times 100}{1877 \times m} \%$
If phosphorus is estimated as $\mathrm{Mg_2} \mathrm{P_2} \mathrm{O_7}$, Percentage of phosphorus $=\dfrac{62 \times m_{1} \times 100}{222}$ :
where, $222 \mathrm{u}$ is the molar mass of $\mathrm{Mg_2} \mathrm{P_2} \mathrm{O_7}$, $m$, the mass of organic compound taken, $m_{1}$, the mass of $\mathrm{Mg_2} \mathrm{P_2} \mathrm{O_7}$ formed and 62, the mass of two phosphorus atoms present in the compound $\mathrm{Mg_2} \mathrm{P_2} \mathrm{O_7}$.
12.10.6 Oxygen
The percentage of oxygen in an organic compound is usually found by difference between the total percentage composition (100) and the sum of the percentages of all other elements. However, oxygen can also be estimated directly as follows:
A definite mass of an organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red-hot coke when all the oxygen is converted to carbon monoxide. This mixture is passed through warm iodine pentoxide $\left(\mathrm{I_2} \mathrm{O_5}\right)$ when carbon monoxide is oxidised to carbon dioxide producing iodine.
Compound $\xrightarrow{\text { heat }} \mathrm{O_2}+ \text{other gaseous product} $
$$2 \mathrm{C}+\mathrm{O_2} \xrightarrow{1373 \mathrm{~K}} 2 \mathrm{CO}] 5 \quad \quad \quad \quad [A]$$
$$\mathrm{I_2} \mathrm{O_5}+5 \mathrm{CO} \longrightarrow \mathrm{I_2}+5 \mathrm{CO_2}] 2 \quad \quad \quad \quad [B]$$
On making the amount of $\mathrm{CO}$ produced in equation (A) equal to the amount of $\mathrm{CO}$ used in equation (B) by multiplying the equations (A) and (B) by 5 and 2 respectively; we find that each mole of oxygen liberated from the compound will produce two moles of carbondioxide.
Thus $88 \mathrm{~g}$ carbon dioxide is obtained if $32 \mathrm{~g}$ oxygen is liberated.
Let the mass of organic compound taken be $m g$ Mass of carbon dioxide produced be $m_{1} g$
$\therefore \mathrm{m_1} \mathrm{~g}$ carbon dioxide is obtained from $\dfrac{32 \times m_{1}}{88} \mathrm{~g} \mathrm{O_2}$
$\therefore$ Percentage of oxygen $=\dfrac{32 \times m_{1} \times 100}{88 \times m} \%$
The percentage of oxygen can be derived from the amount of iodine produced also.
Presently, the estimation of elements in an organic compound is carried out by using microquantities of substances and automatic experimental techniques. The elements, carbon, hydrogen and nitrogen present in a compound are determined by an apparatus known as CHN elemental analyser. The analyser requires only a very small amount of the substance $(1-3 \mathrm{mg})$ and displays the values on a screen within a short time. A detailed discussion of such methods is beyond the scope of this book.
Summary
In this unit, we have learnt some basic concepts in structure and reactivity of organic compounds, which are formed due to covalent bonding. The nature of the covalent bonding in organic compounds can be described in terms of orbitals hybridisation concept, according to which carbon can have $s p^{3}, s p^{2}$ and $s p$ hybridised orbitals. The $s p^{3}, s p^{2}$ and $s p$ hybridised carbons are found in compounds like methane, ethene and ethyne respectively. The tetrahedral shape of methane, planar shape of ethene and linear shape of ethyne can be understood on the basis of this concept. A $s p^{3}$ hybrid orbital can overlap with $1 s$ orbital of hydrogen to give a carbon – hydrogen (C-H) single bond (sigma, $\sigma$ bond). Overlap of a $s p^{2}$ orbital of one carbon with $s p^{2}$ orbital of another results in the formation of a carbon-carbon $\sigma$ bond. The unhybridised $p$ orbitals on two adjacent carbons can undergo lateral (side-by-side) overlap to give a pi ( $\pi$ ) bond. Organic compounds can be represented by various structural formulas. The three dimensional representation of organic compounds on paper can be drawn by wedge and dash formula.
Organic compounds can be classified on the basis of their structure or the functional groups they contain. A functional group is an atom or group of atoms bonded together in a unique fashion and which determines the physical and chemical properties of the compounds. The naming of the organic compounds is carried out by following a set of rules laid down by the International Union of Pure and Applied Chemistry (IUPAC). In IUPAC nomenclature, the names are correlated with the structure in such a way that the reader can deduce the structure from the name.
Organic reaction mechanism concepts are based on the structure of the substrate molecule, fission of a covalent bond, the attacking reagents, the electron displacement effects and the conditions of the reaction. These organic reactions involve breaking and making of covalent bonds. A covalent bond may be cleaved in heterolytic or homolytic fashion. A heterolytic cleavage yields carbocations or carbanions, while a homolytic cleavage gives free radicals as reactive intermediate. Reactions proceeding through heterolytic cleavage involve the complimentary pairs of reactive species. These are electron pair donor known as nucleophile and an electron pair acceptor known as electrophile. The inductive, resonance, electromeric and hyperconjugation effects may help in the polarisation of a bond making certain carbon atom or other atom positions as places of low or high electron densities.
Organic reactions can be broadly classified into following types; substitution, addition, elimination and rearrangement reactions. Purification, qualitative and quantitative analysis of organic compounds are carried out for determining their structures. The methods of purification namely : sublimation, distillation and differential extraction are based on the difference in one or more physical properties. Chromatography is a useful technique of separation, identification and purification of compounds. It is classified into two categories : adsorption and partition chromatography. Adsorption chromatography is based on differential adsorption of various components of a mixture on an adsorbent. Partition chromatography involves continuous partitioning of the components of a mixture between stationary and mobile phases. After getting the compound in a pure form, its qualitative analysis is carried out for detection of elements present in it. Nitrogen, sulphur, halogens and phosphorus are detected by Lassaigne’s test. Carbon and hydrogen are estimated by determining the amounts of carbon dioxide and water produced. Nitrogen is estimated by Dumas or Kjeldahl’s method and halogens by Carius method. Sulphur and phosphorus are estimated by oxidising them to sulphuric and phosphoric acids respectively. The percentage of oxygen is usually determined by difference between the total percentage (100) and the sum of percentages of all other elements present.
Exercise
12.1 What are hybridisation states of each carbon atom in the following compounds ? $\mathrm{CH_2}=\mathrm{C}=\mathrm{O}, \mathrm{CH_3} \mathrm{CH}=\mathrm{CH_2}$, $\left(\mathrm{CH_3}\right)_{2} \mathrm{CO}, \mathrm{CH_2}=\mathrm{CHCN}, \mathrm{C_6} \mathrm{H_6}$
Show Answer
Answer
(i) $\stackrel{1}{C} H_2=\stackrel{2}{C}=0$
C- 1 is $s p^{2}$ hybridised.
C- 2 is sp hybridised.
(ii)
$\stackrel{1}{C} H_3-\stackrel{2}{C} H=\stackrel{3}{C} H_2$
C- 1 is $s p^{3}$ hybridised.
C- 2 is $s p^{2}$ hybridised.
C- 3 is $s p^{2}$ hybridised.
(iii)
C-1 and C-3 are sp³ hybridised.
C- 2 is $s p^{2}$ hybridised.
(iv)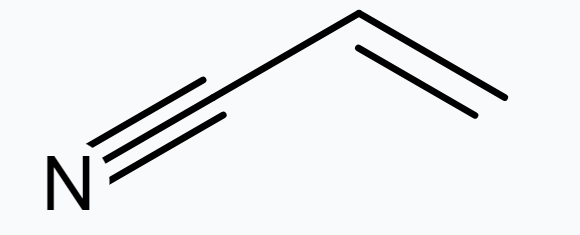
C- 1 is $s p^{2}$ hybridised.
C- 2 is $s p^{2}$ hybridised.
C- 3 is $s p$ hybridised.
(v) $C_6 H_6$
All the 6 carbon atoms in benzene are $s p^{2}$ hybridised.
12.2 Indicate the $\sigma$ and $\pi$ bonds in the following molecules : $\mathrm{C_6} \mathrm{H_6}, \mathrm{C_6} \mathrm{H_12}, \mathrm{CH_2} \mathrm{Cl_2}, \mathrm{CH_2}=\mathrm{C}=\mathrm{CH_2}, \mathrm{CH_3} \mathrm{NO_2}, \mathrm{HCONHCH_3}$
Show Answer
Answer
(i) $C_6 H_6$

There are six C-C sigma ( ${\sigma _{C-C}}$ ) bonds, six C-H sigma ( ${\sigma _{C-H}}$ ) bonds, and three $C=C$ pi $({ }^{C-C})$ resonating bonds in the given https://www.myaischool.in/wp-content/uploads/2025/08/Chapter-12-Organic-Chemistry-Some-Basic-Principles-And-Techniques-12.2.pnghttps://www.myaischool.in/wp-content/uploads/2025/08/Chapter-12-Organic-Chemistry-Some-Basic-Principles-And-Techniques-12.2.pngcompound.
(ii) $C_6 H _{12}$
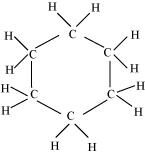
There are six C-C sigma ( $\sigma _{C-C}$ ) bonds and twelve C-H sigma ( $\sigma _{C-H}$ ) bonds in the given compound.
(iii) $CH_2 Cl_2$

There two C-H sigma ( $\sigma _{C-H}$ ) bonds and two C-Cl sigma ( ${\sigma _{C-Cl}}$ ) bonds in the given compound.
(iv) $CH_2=C=CH_2$

There are two C-C sigma ( ${\sigma _{C-C}}$ ) bonds, four C-H sigma ( ${\sigma _{C-H}}$ ) bonds, and two $C=C$ pi ( ${ }^{\pi _{C-C}}$ ) bonds in the given compound.
(v) $CH_3 NO_2$

There are three C-H sigma ( $\sigma _{C-H})$ bonds, one C-N sigma ( $\sigma _{C-N})$ bond, one N-O sigma ( $\sigma _{N-0})$ bond, and one $N=O$ pi ( $.\pi _{N-O})$ bond in the given compound.
(vi) $HCONHCH_3$
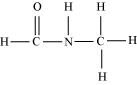
There are two C-N sigma ( $\sigma _{C-N}$ ) bonds, four C-H sigma ( $\sigma _{C-H}$ ) bonds, one N-H sigma bond, and one $C=O$ pi ( $\pi _{C-C}$ ) bond in the given compound.
12.3 Write bond line formulas for : Isopropyl alcohol, 2,3-Dimethylbutanal, Heptan-4-one.
Show Answer
Answer
The bond line formulae of the given compounds are:
(a) Isopropyl alcohol
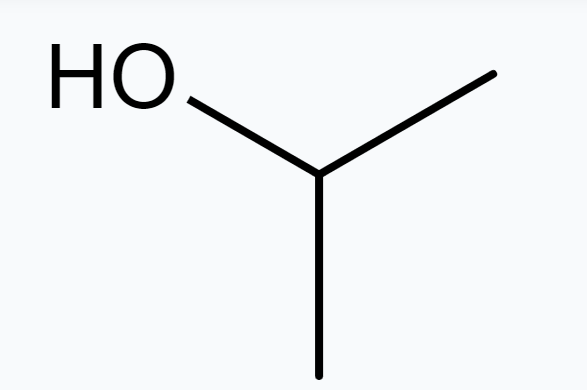
$\Rightarrow$

(b) 2, 3-dimethyl butanal
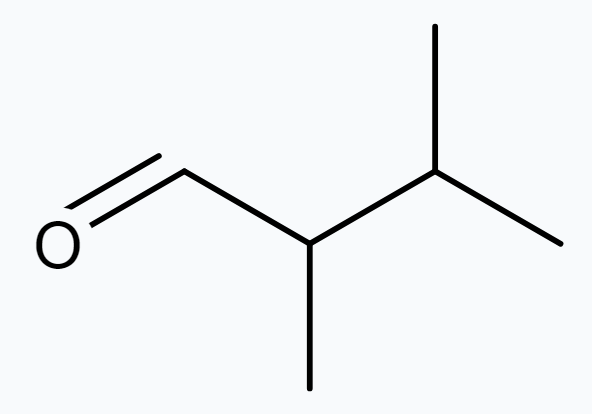
$\Rightarrow$

(c) Heptan-4-one
$
H _3 C-CH _2-CH _2 -\overbrace C^{O}-CH_2-CH_2-CH_3
$
$\Rightarrow$

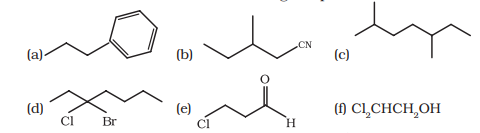
12.4 Give the IUPAC names of the following compounds :
Show Answer
Answer
(a)

1-phenyl propane
(b)

3-methylpentanenitrile
(c)

2, 5-dimethyl heptane
(d)

3-bromo-3-chloroheptane
(e)

3-chloropropanal
(f) $Cl_2 CHCH_2 OH$
1, 1-dichloro-2-ethanol
12.5 Which of the following represents the correct IUPAC name for the compounds concerned ?
(a) 2,2-Dimethylpentane or 2-Dimethylpentane
(b) 2,4,7-Trimethyloctane or 2,5,7-Trimethyloctane
(c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane
(d) But-3-yn-1-ol or But-4-ol-1-yne.
Show Answer
Answer
(a) The prefix diin the IUPAC name indicates that two identical substituent groups are present in the parent chain. Since two methyl groups are present in the C-2 of the parent chain of the given compound, the correct IPUAC name of the given compound is 2, 2-dimethylpentane.
(b) Locant number 2, 4, 7 is lower than 2, 5, 7. Hence, the IUPAC name of the given compound is 2, 4, 7trimethyloctane.
(c) If the substituents are present in the equivalent position of the parent chain, then the lower number is given to the one that comes first in the name according to the alphabetical order. Hence, the correct IUPAC name of the given compound is 2-chloro-4-methylpentane.
(d) Two functional groups – alcoholic and alkyne – are present in the given compound. The principal functional group is the alcoholic group. Hence, the parent chain will be suffixed with ol. The alkyne group is present in the C-3 of the parent chain. Hence, the correct IUPAC name of the given compound is But-3-yn-1-ol.
12.6 Draw formulas for the first five members of each homologous series beginning with the
following compounds. (a) $\mathrm{H}-\mathrm{COOH}$ (b) $\mathrm{CH_3} \mathrm{COCH_3}$ (c) $\mathrm{H}-\mathrm{CH}=\mathrm{CH_2}$
Show Answer
Answer
The first five members of each homologous series beginning with the given compounds are shown as follows:
(a)
$H-COOH$ : Methanoic acid
$CH_3-COOH$ : Ethanoic acid
$CH_3-CH_2-COOH$ : Propanoic acid
$CH_3-CH_2-CH_2-COOH$ : Butanoic acid
$CH_3-CH_2-CH_2-CH_2-COOH$ : Pentanoic acid
(b)
$CH_3 COCH_3$ : Propanone
$CH_3 COCH_2 CH_3$ : Butanone
$CH_3 COCH_2 CH_2 CH_3$ : Pentan-2-one
$CH_3 COCH_2 CH_2 CH_2 CH_3$ : Hexan-2-one
$CH_3 COCH_2 CH_2 CH_2 CH_2 CH_3$ : Heptan-2-one
(c)
$H-CH=CH_2$ : Ethene
$CH_3-CH=CH_2$ : Propene
$CH_3-CH_2-CH=CH_2:$ 1-Butene
$CH_3-CH_2-CH_2-CH=CH_2$ : 1-Pentene
$CH_3-CH_2-CH_2-CH_2-CH=CH_2: 1-Hexene$
12.7 Give condensed and bond line structural formulas and identify the functional group(s)
present, if any, for :
(a) 2,2,4-Trimethylpentane
(b) 2-Hydroxy-1,2,3-propanetricarboxylic acid
(c) Hexanedial
Show Answer
Answer
(a) 2, 2, 4-trimethylpentane
Condensed formula:
$(CH_3)_2 CHCH_2 C(CH_3)_3$
Bond line formula:

(b) 2-hydroxy-1, 2, 3-propanetricarboxylic acid Condensed Formula:
$(COOH) CH_2 C(OH)(COOH) CH_2(COOH)$
Bond line formula:
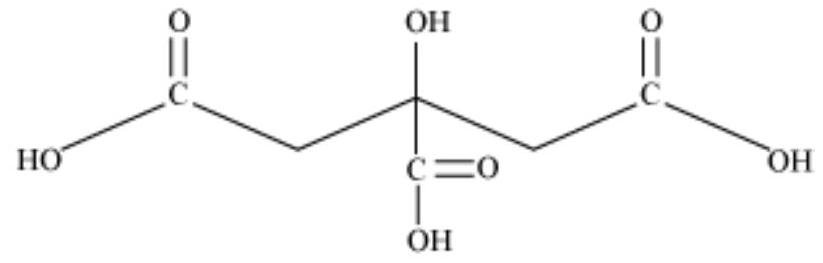
The functional groups present in the given compound are carboxylic acid (-COOH) and alcoholic (-OH) groups.
(c) Hexanedial
Condensed Formula:
$(CHO)(CH_2)_4(CHO)$
Bond line Formula:

The functional group present in the given compound is aldehyde $(-CHO)$.
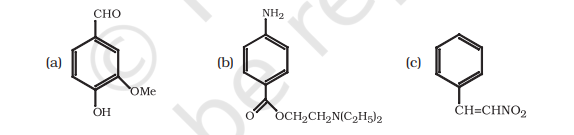
12.8 Identify the functional groups in the following compounds
Show Answer
Answer
The functional groups present in the given compounds are:
(a) Aldehyde (-CHO),
Hydroxyl(-OH),
Methoxy (-OMe),
$C=C$ double bond $(-\stackrel{1}{C}=\stackrel{1}{C}-)$
(b) Amino (- $NH_2$ ); primary amine,
Ester (-O-CO-),
Triethylamine $(N(C_2 H_5)_2)$; tertiary amine
(c) Nitro (– $NO_2$ ),
$C=C$ double bond $(-C=C-)$
12.9 Which of the two: $\mathrm{O_2} \mathrm{NCH_2} \mathrm{CH_2} \mathrm{O}^{-}$or $\mathrm{CH_3} \mathrm{CH_2} \mathrm{O}^{-}$is expected to be more stable and why?
Show Answer
Answer
$NO_2$ group is an electron-withdrawing group. Hence, it shows -I effect. By withdrawing the electrons toward it, the $NO_2$ group decreases the negative charge on the compound, thereby stabilising it. On the other hand, ethyl group is an electron-releasing group. Hence, the ethyl group shows +1 effect. This increases the negative charge on the compound, thereby destabilising it. Hence, $O_2 NCH_2 CH_2 O$ is expected to be more stable than $CH_3 CH_2 O$.
12.10 Explain why alkyl groups act as electron donors when attached to a $\pi$ system.
Show Answer
Answer
When an alkyl group is attached to a , $\neg$ system, it acts as an electron-donor group by the process of hyperconjugation. To understand this concept better, let us take the example of propene.
In hyperconjugation, the sigma electrons of the $C-H$ bond of an alkyl group are delocalised. This group is directly attached to an atom of an unsaturated system. The delocalisation occurs because of a partial overlap of a $s p^{3}$-ssigma bond orbital with an empty $p$ orbital of the – $\neg$ bond of an adjacent carbon atom.
The process of hyperconjugation in propene is shown as follows:

This type of overlap leads to a delocalisation (also known as no-bond resonance) of the – $\neg$ electrons, making the molecule more stable.

IV
III
12.11 Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) $\mathrm{C_6} \mathrm{H_5} \mathrm{OH}$
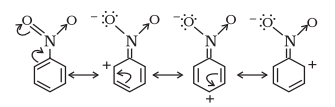
(b) $\mathrm{C_6} \mathrm{H_5} \mathrm{NO_2}$
(c) $\mathrm{CH_3} \mathrm{CH}=\mathrm{CHCHO}$
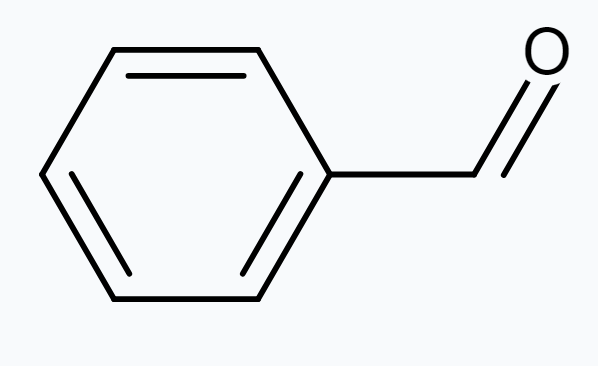
(d) $\mathrm{C_6} \mathrm{H_5}-\mathrm{CHO}$
(e) $\mathrm{C_6} \mathrm{H_5}-\stackrel{+}{\mathrm{C}} \mathrm{H_2}$
(f) $\mathrm{CH_3} \mathrm{CH}=\mathrm{CH} \stackrel{+}{\mathrm{C}} \mathrm{H_2}$
Show Answer
Answer
(a) The structure of $C_6 H_5 OH$ is:
The resonating structures of phenol are represented as:

(b) The structure of $C_6 H_5 NO_2$ is:

The resonating structures of nitro benzene are represented as:
(c) $CH_3 CH=CH-CHO$
The resonating structures of the given compound are represented as:
1
II
(d) The structure of $C_6 H_5 CHO$ is:

The resonating structures of benzaldehyde are represented as:

(e) $C_6 H_5 CH_2^{-}$
The resonating structures of the given compound are:

(f) $CH_3 CH=CHCH_2^{-}$
The resonating structures of the given compound are:

12.12 What are electrophiles and nucleophiles ? Explain with examples.
Show Answer
Answer
An electrophile is a reagent that takes away an electron pair. In other words, an electron-seeking reagent is called an electrophile $(E^{+})$. Electrophiles are electron-deficient and can receive an electron pair.
Carbocations $(CH_3 CH_2^{+})$and neutral molecules having functional groups such as carbonyl group $(https://temp-public-img-folder.s3.ap-south-1.amazonaws.com/sathee.prutor.images/
C=0$ ) are examples of electrophiles.
A nulceophile is a reagent that brings an electron pair. In other words, a nucleus-seeking reagent is called a nulceophile (Nu:).
For example: $OH^{-}, NC^{-} e^{e}$, carbanions $(R_3 C^{-})$, etc.
Neutral molecules such as $H_2-$ and ammonia also act as nulceophiles because of the presence of a lone pair.
12.13 Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles:
(a) $\mathrm{CH_3} \mathrm{COOH}+\mathbf{H O}^{-} \rightarrow \mathrm{CH_3} \mathrm{COO}^{-}+\mathrm{H_2} \mathrm{O}$
(b) $\mathrm{CH_3} \mathrm{COCH_3}+\overline{\mathbf{C N}} \rightarrow\left(\mathrm{CH_3}\right)_{2} \mathrm{C}(\mathrm{CN})(\mathrm{OH})$
(c) $\mathrm{C_6} \mathrm{H_6}+\mathbf{C H_3} \stackrel{+}{\mathbf{C}} \mathbf{O} \rightarrow \mathrm{C_6} \mathrm{H_5} \mathrm{COCH_3}$
Show Answer
Answer
Electrophiles are electron-deficient species and can receive an electron pair. On the other hand, nucleophiles are electron-rich species and can donate their electrons.
(a) $CH_3 COOH+HO^{-} \longrightarrow CH_3 COO^{-}+H_2 O$
Here, $HO^{-} e$ acts as a nucleophile as it is an electron-rich species, i.e., it is a nucleus-seeking species.
(b) $CH_3 COCH_3+\stackrel{-}{\mathbf{C}} \mathbf{N} \longrightarrow(CH_3)_2 C(CN)+(OH)$
Here, $- CN$ acts as a nucleophile as it is an electron-rich species, i.e., it is a nucleus-seeking species.
(c) $C_6 H_5+\mathbf{C H}_3 \stackrel{+}{\mathbf{C}} O \longrightarrow C_6 H_5 COCH_3$
Here, $CH_3 \stackrel{+}{C} O$ acts as an electrophile as it is an electron-deficient species.
12.14 Classify the following reactions in one of the reaction type studied in this unit.
(a) $\mathrm{CH_3} \mathrm{CH_2} \mathrm{Br}+\mathrm{HS}^{-} \rightarrow \mathrm{CH_3} \mathrm{CH_2} \mathrm{SH}+\mathrm{Br}^{-}$
(b) $\left(\mathrm{CH_3}\right)_2 \mathrm{C}=\mathrm{CH_2}+\mathrm{HCI} \rightarrow\left(\mathrm{CH_3}\right)_2 \mathrm{CIC}-\mathrm{CH_3}$
(c) $\mathrm{CH_3} \mathrm{CH_2} \mathrm{Br}+\mathrm{HO}^{-} \rightarrow \mathrm{CH_2}=\mathrm{CH_2}+\mathrm{H_2} \mathrm{O}+\mathrm{Br}^{-}$
(d) $\left(\mathrm{CH_3}\right)_3 \mathrm{C}-\mathrm{CH_2} \mathrm{OH}+\mathrm{HBr} \rightarrow\left(\mathrm{CH_3}\right)_2 \mathrm{CBrCH_2} \mathrm{CH_2} \mathrm{CH_3}+\mathrm{H_2} \mathrm{O}$
Show Answer
Answer
(a) It is an example of substitution reaction as in this reaction the bromine group in bromoethane is substituted by the -SH group.
(b) It is an example of addition reaction as in this reaction two reactant molecules combine to form a single product.
(c) It is an example of elimination reaction as in this reaction hydrogen and bromine are removed from bromoethane to give ethene.
(d) In this reaction, substitution takes place, followed by a rearrangement of atoms and groups of atoms.
12.15 What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
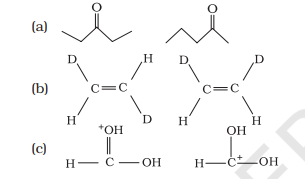
Show Answer
Answer
(a) Compounds having the same molecular formula but with different structures are called structural isomers. The given compounds have the same molecular formula but they differ in the position of the functional group (ketone group).

In structure I, ketone group is at the C-3 of the parent chain (hexane chain) and in structure II, ketone group is at the $C-2$ of the parent chain (hexane chain). Hence, the given pair represents structural isomers.
(b) Compounds having the same molecular formula, the same constitution, and the sequence of covalent bonds, but with different relative position of their atoms in space are called geometrical isomers.
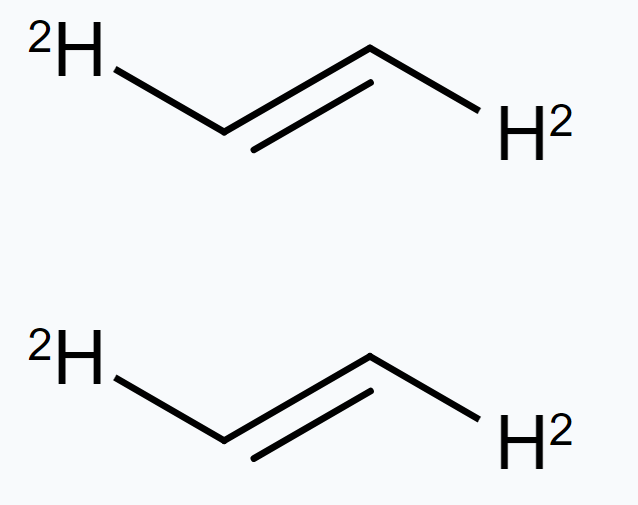
In structures I and II, the relative position of Deuterium (D) and hydrogen $(H)$ in space are different. Hence, the given pairs represent geometrical isomers.
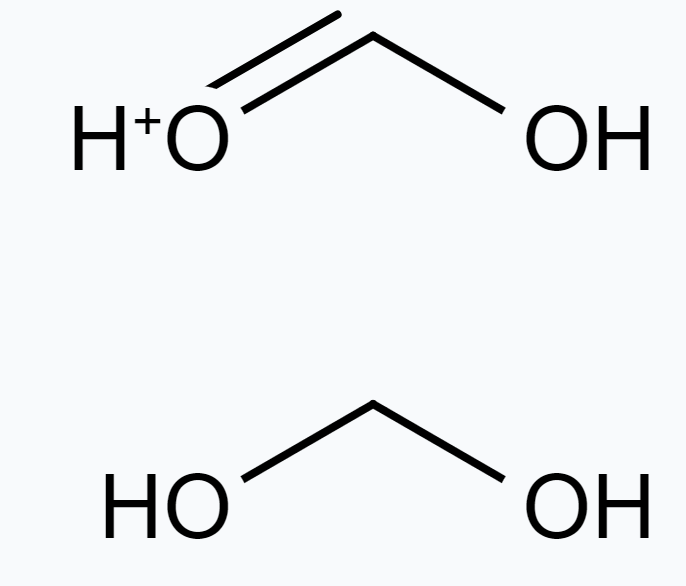
(c) The given structures are canonical structures or contributing structures. They are hypothetical and individually do not represent any real molecule. Hence, the given pair represents resonance structures, called resonance isomers.
12.16 For the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.

Show Answer
Answer
(a) The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as

It is an example of homolytic cleavage as one of the shared pair in a covalent bond goes with the bonded atom. The reaction intermediate formed is a free radical.
(b) The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as

It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the carbon of propanone. The reaction intermediate formed is carbanion.
(c) The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as

It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the bromine ion. The reaction intermediate formed is a carbocation.
(d) The bond cleavage using curved-arrows to show the electron flow of the given reaction can be represented as
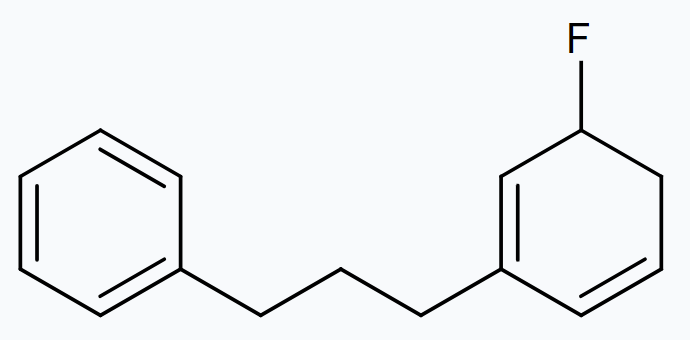
It is a heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with one of the fragments. The intermediate formed is a carbocation.
12.17 Explain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) $\mathrm{Cl_3} \mathrm{CCOOH}>\mathrm{Cl_2} \mathrm{CHCOOH}>\mathrm{ClCH_2} \mathrm{COOH}$
(b) $\mathrm{CH_3} \mathrm{CH_2} \mathrm{COOH}>\left(\mathrm{CH_3}\right)_2 \mathrm{CHCOOH}>\left(\mathrm{CH_3}\right)_3 \mathrm{C} . \mathrm{COOH}$
Show Answer
Answer
Inductive effect
The permanent displacement of sigma ( $\tilde{A} E^{\prime}$ ) electrons along a saturated chain, whenever an electron withdrawing or electron donating group is present, is called inductive effect.
Inductive effect could be $+I$ effect or $-I$ effect. When an atom or group attracts electrons towards itself more strongly than hydrogen, it is said to possess – I effect. For example,
$F \longrightarrow CH_2 \longleftarrow CH_2 \backsim CH_2 \backsim CH_3$
When an atom or group attracts electrons towards itself less strongly than hydrogen, it is said to possess +1 effect. For example,
$CH_3 \longrightarrow CH_2 \to Cl$
Electrometric effect
It involves the complete transfer of the shared pair of – $\urcorner$ electrons to either of the two atoms linked by multiple bonds in the presence of an attacking agent. For example,
Electrometric effect could be $+E$ effect or $-E$ effect.
- E effect: When the electrons are transferred towards the attacking reagent
- E effect: When the electrons are transferred away from the attacking reagent
(a) $Cl_3 CCOOH>Cl_2 CHCOOH>ClCH_2 COOH$
The order of acidity can be explained on the basis of Inductive effect (- I effect). As the number of chlorine atoms increases, the – I effect increases. With the increase in – I effect, the acid strength also increases accordingly.
(b) $CH_3 CH_2 COOH>(CH_3)_2 CHCOOH>(CH_3)_3 C . COOH$
The order of acidity can be explained on the basis of inductive effect (+ I effect). As the number of alkyl groups increases, the $+I$ effect also increases. With the increase in $+I$ effect, the acid strength also increases accordingly.

12.18 Give a brief description of the principles of the following techniques taking an example in each case.
(a) Crystallisation
(b) Distillation
(c) Chromatography
Show Answer
Answer
(a) Crystallisation
Crystallisation is one of the most commonly used techniques for the purification of solid organic compounds.
Principle: It is based on the difference in the solubilites of the compound and the impurities in a given solvent. The impure compound gets dissolved in the solvent in which it is sparingly soluble at room temperature, but appreciably soluble at higher temperature. The solution is concentrated to obtain a nearly saturated solution. On cooling the solution, the pure compound crystallises out and is removed by filtration.
For example, pure aspirin is obtained by recrystallising crude aspirin. Approximately $2-4 g$ of crude aspirin is dissolved in about $20 mL$ of ethyl alcohol. The solution is heated (if necessary) to ensure complete dissolution. The solution is then left undisturbed until some crystals start to separate out. The crystals are then filtered and dried.
(b) Distillation
This method is used to separate volatile liquids from non-volatile impurities or a mixture of those liquids that have a sufficient difference in their boiling points.
Principle: It is based on the fact that liquids having different boiling points vapourise at different temperatures. The vapours are then cooled and the liquids so formed are collected separately.
For example, a mixture of chloroform ( $b . p=334 K$ ) and aniline ( $b . p=457 K$ ) can be separated by the method of distillation. The mixture is taken in a round bottom flask fitted with a condenser. It is then heated. Chloroform, being more volatile, vaporizes first and passes into the condenser. In the condenser, the vapours condense and chloroform trickles down. In the round bottom flask, aniline is left behind.
(c) Chromatography
It is one of the most useful methods for the separation and purification of organic compounds.
Principle: It is based on the difference in movement of individual components of a mixture through the stationary phase under the influence of mobile phase.
For example, a mixture of red and blue ink can be separated by chromatography. A drop of the mixture is placed on the chromatogram. The component of the ink, which is less adsorbed on the chromatogram, moves with the mobile phase while the less adsorbed component remains almost stationary.
12.19 Describe the method, which can be used to separate two compounds with different solubilities in a solvent $\mathrm{S}$.
Show Answer
Answer
Fractional crystallisation is the method used for separating two compounds with different solubilities in a solvent $S$. The process of fractional crystallisation is carried out in four steps.
(a) Preparation of the solution: The powdered mixture is taken in a flask and the solvent is added to it slowly and stirred simultaneously. The solvent is added till the solute is just dissolved in the solvent. This saturated solution is then heated.
(b) Filtration of the solution: The hot saturated solution is then filtered through a filter paper in a China dish.
(c) Fractional crystallisation: The solution in the China dish is now allowed to cool. The less soluble compound crystallises first, while the more soluble compound remains in the solution. After separating these crystals from the
mother liquor, the latter is concentrated once again. The hot solution is allowed to cool and consequently, the crystals of the more soluble compound are obtained.
(d) Isolation and drying: These crystals are separated from the mother liquor by filtration. Finally, the crystals are dried.
12.20 What is the difference between distillation, distillation under reduced pressure and steam distillation?
Show Answer
Answer
The differences among distillation, distillation under reduced pressure,
and steam distillation are given in the following table.
| Distillation | Distillation under reduced pressure | Steam distillation | |
|---|---|---|---|
| 1.It is used for the purification of compounds that are associated with non-volatile impurities or those liquids, which do not decompose on boiling. In other words, distillation is used to separate volatile liquids from non-volatile impurities or a mixture of those liquids that have sufficient difference in boiling points. | This method is used to purify a liquid that tends to decompose on boiling. Under pressure, the liquid will boil at a low temperature than its boiling point and will, therefore, not decompose. | compound, which is steam volatile and immiscible in water. On passing steam, the compound gets condensed to water. After some time, the mixture of water and liquid starts to boil and passes through the condenser. This condensed mixture of water and liquid is then separated by using a separating funnel. | |
| 2. | Mixture of petrol and kerosene is separated by this method. | Glycerol is purified by this method. It boils with decomposition at a temperature of 593 K. At a reduced pressure, it boils at 453 K without decomposition. | A mixture of water and aniline is separated by steam distillation. |
12.21 Discuss the chemistry of Lassaigne’s test.
Show Answer
Answer
Lassaigne’s test
This test is employed to detect the presence of nitrogen, sulphur, halogens, and phosphorous in an organic compound. These elements are present in the covalent form in an organic compound. These are converted into the ionic form by fusing the compound with sodium metal.
$
\begin{aligned}
& Na+C+N \xrightarrow{\Delta} NaCN \\
& 2 Na+S \xrightarrow{\Delta} Na_2 S \\
& Na+X \xrightarrow{\Delta} NaX \\
& (X=Cl, Br, I)
\end{aligned}
$
The cyanide, sulphide, and halide of sodium formed are extracted from the fused mass by boiling it in distilled water. The extract so obtained is called Lassaigne’s extract. This Lassaigne’s extract is then tested for the presence of nitrogen, sulphur, halogens, and phosphorous.
(a) Test for nitrogen

Prussian blue colour
(Ferriferro cyanide)
Chemistry of the test
In the Lassaigne’s test for nitrogen in an organic compound, the sodium fusion extract is boiled with iron (II) sulphate and then acidified with sulphuric acid. In the process, sodium cyanide first reacts with iron (II) sulphate and forms sodium hexacyanoferrate (II). Then, on heating with sulphuric acid, some iron (II) gets oxidised to form iron (III) hexacyanoferrate (II), which is Prussian blue in colour. The chemical equations involved in the reaction can be represented as
$
\begin{aligned}
& 6 CN^{-}+Fe^{2+} \longrightarrow[Fe(CN)_6]^{4-} \\
& 3[Fe(CN)_6]^{4-}+4 Fe^{3+} \xrightarrow{x H_2 O} Fe_4[Fe(CN)_6]_3 x H_2 O
\end{aligned}
$
Prussian blue colour
(b) Test for sulphur
(i) Lassaigne’s extract + Lead acetate $\xrightarrow{\text{ acetic acid }}$ Black precipitate
Chemistry of the test
In the Lassaigne’s test for sulphur in an organic compound, the sodium fusion extract is acidified with acetic acid and then lead acetate is added to it. The precipitation of lead sulphide, which is black in colour, indicates the presence of sulphur in the compound.
$S^{2-}+Pb^{2+} \longrightarrow PbS$
(Black)
(ii) Lassaigne’s extract + Sodium nitroprusside $\longrightarrow$ Violet colour
Chemistry of the test
The sodium fusion extract is treated with sodium nitroprusside. Appearance of violet colour also indicates the presence of sulphur in the compound.
$
\begin{aligned}
& S^{2-}+[.Fe(CN)_5 NO]^{2-} \longrightarrow \\
&(\text{ Violet) }
\end{aligned}
$
If in an organic compound, both nitrogen and sulphur are present, then instead of $NaCN$, formation of NaSCN takes place.
$Na+C+N+S$ Ã
This NaSCN (sodium thiocyanate) gives a blood red colour. Prussian colour is not formed due to the absence of free cyanide ions.
$
Fe^{3+}+SCN^{-} \longrightarrow[Fe(SCN)]^{2+}
$
(Blood red)
(c) Test for halogens

Chemistry of the test
In the Lassaigne’s test for halogens in an organic compound, the sodium fusion extract is acidified with nitric acid and then treated with silver nitrate.
$
\begin{aligned}
& X^{-}+Ag^{+} \longrightarrow AgX \\
&(X=Cl, Br, I)
\end{aligned}
$
If nitrogen and sulphur both are present in the organic compound, then the Lassaigne’s extract is boiled to expel nitrogen and sulphur, which would otherwise interfere in the test for halogens.
12.22 Differentiate between the principle of estimation of nitrogen in an organic compound by (i) Dumas method and (ii) Kjeldahl’s method.
Show Answer
Answer
In Dumas method, a known quantity of nitrogen containing organic compound is heated strongly with excess of copper oxide in an atmosphere of carbon dioxide to produce free nitrogen in addition to carbon dioxide and water. The chemical equation involved in the process can be represented as
$
C x HyNz+(2 x+y / 2) CuO \longrightarrow x CO_2+y / 2 H_2 O+z / 2 N_2+(2 x+y / 2) Cu
$
The traces of nitrogen oxides can also be produced in the reaction, which can be reduced to dinitrogen by passing the gaseous mixture over a heated copper gauge. The dinitrogen produced is collected over an aqueous solution of potassium hydroxide. The volume of nitrogen produced is then measured at room temperature and atmospheric pressure.
On the other hand, in Kjeldahl’s method, a known quantity of nitrogen containing organic compound is heated with concentrated sulphuric acid. The nitrogen present in the compound is quantitatively converted into ammonium sulphate. It is then distilled with excess of sodium hydroxide. The ammonia evolved during this process is passed into a known volume of $H_2 SO_4$. The chemical equations involved in the process are

The acid that is left unused is estimated by volumetric analysis (titrating it against a standard alkali) and the amount of ammonia produced can be determined. Thus, the percentage of nitrogen in the compound can be estimated. This method cannot be applied to the compounds, in which nitrogen is present in a ring structure, and also not applicable to compounds containing nitro and azo groups.
12.23 Discuss the principle of estimation of halogens, sulphur and phosphorus present in an organic compound.
Show Answer
Answer
Estimation of halogens
Halogens are estimated by the Carius method. In this method, a known quantity of organic compound is heated with fuming nitric acid in the presence of silver nitrate, contained in a hard glass tube called the Carius tube, taken in a furnace. Carbon and hydrogen that are present in the compound are oxidized to form $CO_2$ and $H_2 O$ respectively and the halogen present in the compound is converted to the form of $AgX$. This $AgX$ is then filtered, washed, dried, and weighed.
Let the mass of organic compound be $m g$.
Mass of $AgX$ formed $=m_1 g$
1 mol of Agx contains 1 mol of $X$.
Therefore,
Mass of halogen inm $I_1 g$ of $AgX$
$
=\frac{\text{ Atomic mass of } X \times m_1 g}{\text{ Molecular mass of } AgX}
$
Thus, $%$ of halogen will be $=\frac{\text{ Atomic mass of } X \times m_1 \times 100}{\text{ Molecular mass of } AgX \times m}$
Estimation of Sulphur
In this method, a known quantity of organic compound is heated with either fuming nitric acid or sodium peroxide in a hard glass tube called the Carius tube. Sulphur, present in the compound, is oxidized to form sulphuric acid. On addition of excess of barium chloride to it, the precipitation of barium sulphate takes place. This precipitate is then filtered, washed, dried, and weighed.
Let the mass of organic compound be $m g$.
Mass of $BaSO_4$ formed $=m_1 g$
$1 mol^{2}$ of $BaSO_4=233 g BaSO_4=32 g$ of Sulphur
Therefore, $m_1 g$ of $BaSO_4$ contains $\frac{32 \times m_1}{233} g$ of sulphur.
Thus, percentage of sulphur $=\frac{32 \times m_1 \times 100}{233 \times m}$
Estimation of phosphorus
In this method, a known quantity of organic compound is heated with fuming nitric acid. Phosphorus, present in the compound, is oxidized to form phosphoric acid. By adding ammonia and ammonium molybdate to the solution, phosphorus can be precipitated as ammonium phosphomolybdate.
Phosphorus can also be estimated by precipitating it as $MgNH_4 PO_4$ by adding magnesia mixture, which on ignition yields $Mg_2 P_2 O_7$.
Let the mass of organic compound be $m g$.
Mass of ammonium phosphomolybdate formed $=m_1 g$
Molar mass of ammonium phosphomolybdate $=1877 g$
Thus, percentage of phosphorus $=\frac{31 \times m_1 \times 100}{1877 \times m} %$
If $P$ is estimated as $Mg_2 P_2 O_7$,
Then, percentage of phosphorus $=\frac{62 \times m_1 \times 100}{222 \times m} %$
12.24 Explain the principle of paper chromatography.
Show Answer
Answer
In paper chromatography, chromatography paper is used. This paper contains water trapped in it, which acts as the stationary phase. On the base of this chromatography paper, the solution of the mixture is spotted. The paper strip is then suspended in a suitable solvent, which acts as the mobile phase. This solvent rises up the chromatography
paper by capillary action and in the procedure, it flows over the spot. The components are selectively retained on the paper (according to their differing partition in these two phases). The spots of different components travel with the mobile phase to different heights. The paper so obtained (shown in the given figure) is known as a chromatogram.

12.25 Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens?
Show Answer
Answer
While testing the Lassaigne’s extract for the presence of halogens, it is first boiled with dilute nitric acid. This is done to decompose $NaCN$ to $HCN$ and $Na_2 S$ to $H_2 S$ and to expel these gases. That is, if any nitrogen and sulphur are present in the form of $NaCN$ and $Na_2 S$, then they are removed. The chemical equations involved in the reaction are represented as
$
\begin{aligned}
& NaCN+HNO_3 \longrightarrow NaNO_3+HCN \\
& Na_2 S+2 HNO_3 \longrightarrow 2 NaNO_3+H_2 S
\end{aligned}
$
12.26 Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens.
Show Answer
Answer
Nitrogen, sulphur, and halogens are covalently bonded in organic compounds. For their detection, they have to be first converted to ionic form. This is done by fusing the organic compound with sodium metal. This is called “Lassaigne’s test”. The chemical equations involved in the test are
$
\begin{aligned}
& Na+C+N \longrightarrow NaCN \\
& Na+S+C+N \longrightarrow NaSCN \\
& 2 Na+S \longrightarrow Na_2 S \\
& Na+X \longrightarrow NaX \\
&(X=Cl, Br, I)
\end{aligned}
$
Carbon, nitrogen, sulphur, and halogen come from organic compounds.
12.27 Name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor.
Show Answer
Answer
The process of sublimation is used to separate a mixture of camphor and calcium sulphate. In this process, the sublimable compound changes from solid to vapour state without passing through the liquid state. Camphor is a sublimable compound and calcium sulphate is a non-sublimable solid. Hence, on heating, camphor will sublime while calcium sulphate will be left behind.
12.28 Explain, why an organic liquid vaporises at a temperature below its boiling point in its steam distillation?
Show Answer
Answer
In steam distillation, the organic liquid starts to boil when the sum of vapour pressure due to the organic liquid $(p_1)$ and the vapour pressure due to water $(p_2)$ becomes equal to atmospheric pressure $(p)$, that is, $p=p_1+p_2$
Since $p_1<p_2$, organic liquid will vapourise at a lower temperature than its boiling point.
12.29 Will $\mathrm{CCl_4}$ give white precipitate of $\mathrm{AgCl}$ on heating it with silver nitrate? Give reason for your answer.
Show Answer
Answer
$CCl_4$ will not give the white precipitate of $AgCl$ on heating it with silver nitrate. This is because the chlorine atoms are covalently bonded to carbon in $CCl_4$. To obtain the precipitate, it should be present in ionic form and for this, it is necessary to prepare the Lassaigne’s extract of $CCl_4$.
12.30 Why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in an organic compound?
Show Answer
Answer
Carbon dioxide is acidic in nature and potassium hydroxide is a strong base. Hence, carbon dioxide reacts with potassium hydroxide to form potassium carbonate and water as
$2 KOH+CO_2 \longrightarrow K_2 CO_3+H_2 O$
Thus, the mass of the U-tube containing $KOH$ increases. This increase in the mass of U-tube gives the mass of $CO_2$ produced. From its mass, the percentage of carbon in the organic compound can be estimated.
12.31 Why is it necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test?
Show Answer
Answer
Although the addition of sulphuric acid will precipitate lead sulphate, the addition of acetic acid will ensure a complete precipitation of sulphur in the form of lead sulphate due to common ion effect. Hence, it is necessary to use acetic acid for acidification of sodium extract for testing sulphur by lead acetate test.
12.32 An organic compound contains $69 \%$ carbon and $4.8 \%$ hydrogen, the remainder being oxygen. Calculate the masses of carbon dioxide and water produced when $0.20 \mathrm{~g}$ of this substance is subjected to complete combustion.
Show Answer
Answer
Percentage of carbon in organic compound $=69 %$
That is, $100 g$ of organic compound contains $69 g$ of carbon.
$\therefore 0.2 g$ of organic compound will contain $100=0.138 g$ of $C$
$
=\frac{69 \times 0.2}{100}=0.138 g \text{ of } C
$
Molecular mass of carbon dioxide, $CO_2=44 g$
That is, $12 g$ of carbon is contained in $44 g$ of $CO_2$.
$
\text{ Therefore, } 0.138 g \text{ of carbon will be contained in } \frac{44 \times 0.138}{12}=0.506 g \text{ of } CO_2
$
Thus, $0.506 g$ of $CO_2$ will be produced on complete combustion of $0.2 g$ of organic compound.
Percentage of hydrogen in organic compound is 4.8.
i.e., $100 g$ of organic compound contains $4.8 g$ of hydrogen.
Therefore, $0.2 g$ of organic compound will contain
$
\frac{4.8 \times 0.2}{100}=0.0096 g \text{ of } H
$
It is known that molecular mass of water $(H_2 O)$ is $18 g$.
Thus, $2 g$ of hydrogen is contained in $18 g$ of water.
$\therefore 0.0096 g$ of hydrogen will be contained in $\frac{18 \times 0.0096}{2}=0.0864 g$ of water
Thus, $0.0864 g$ of water will be produced on complete combustion of $0.2 g$ of the organic compound.
12.33 A sample of $0.50 \mathrm{~g}$ of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in $50 \mathrm{ml}$ of $0.5 \mathrm{M} \mathrm{H_2} \mathrm{SO_4}$. The residual acid required $60 \mathrm{~mL}$ of $0.5 \mathrm{M}$ solution of $\mathrm{NaOH}$ for neutralisation. Find the percentage composition of nitrogen in the compound.
Show Answer
Answer
Given that, total mass of organic compound $=0.50 g$
$60 mL$ of $0.5 M$ solution of $NaOH$ was required by residual acid for neutralisation.
$60 mL$ of $0.5 M NaOH$ solution $=\frac{60}{2} mL$ of $0.5 M H_2 SO_4=30 mL$ of $0.5 M H_2 SO_4$
$
=\frac{60}{2} mL \text{ of } 0.5 M
$
$\therefore$ Acid consumed in absorption of evolved ammonia is (50âє”30) mL $=20 mL$
Again, $20 mL$ of $0.5 MH_2 SO_4=40 mL$ of $0.5 MNH_3$
Also, since $1000 mL$ of $1 MNH_3$ contains $14 g$ of nitrogen,
$\therefore 40 mL$ of $0.5 M NH_3$ will contain $\frac{14 \times 40}{1000} \times 0.5=0.28 g$ of N
Therefore, percentage of nitrogen in $0.50 g$ of organic compound $=\frac{0.28}{0.50} \times 100=56 %$
12.34 $ 0.3780 \mathrm{~g}$ of an organic chloro compound gave $0.5740 \mathrm{~g}$ of silver chloride in Carius estimation. Calculate the percentage of chlorine present in the compound.
Show Answer
Answer
Given that,
Mass of organic compound is $0.3780 g$.
Mass of $AgCl$ formed $=0.5740 g$
$1 mol$ of $AgCl$ contains $1 mol$ of $Cl$.
Thus, mass of chlorine in $0.5740 g$ of $AgCl$
$=\frac{35.5 \times 0.5740}{143.32}$
$=0.1421 g$
$\therefore$ Percentage of chlorine $=\frac{0.1421}{0.3780} \times 100=37.59 %$
Hence, the percentage of chlorine present in the given organic chloro compound is $37.59 %$
12.35 In the estimation of sulphur by Carius method, $0.468 \mathrm{~g}$ of an organic sulphur compound afforded $0.668 \mathrm{~g}$ of barium sulphate. Find out the percentage of sulphur in the given compound.
Show Answer
Answer
Total mass of organic compound $=0.468 g$ [Given]
Mass of barium sulphate formed $=0.668 g$ [Given]
$1 mol$ of $BaSO_4=233 g$ of $BaSO_4=32 g$ of sulphur
Thus, $0.668 g$ of $BaSO_4$ contains $\frac{32 \times 0.668}{233} g$ of sulphur $=0.0917 g$ of sulphur
Therefore, percentage of sulphur $=\frac{0.0197}{0.468} \times 100=19.59 %$
Hence, the percentage of sulphur in the given compound is $19.59 %$.
12.36 In the organic compound $\mathrm{CH_2}=\mathrm{CH}-\mathrm{CH_2}-\mathrm{CH_2}-\mathrm{C} \equiv \mathrm{CH}$, the pair of hydridised orbitals involved in the formation of: $\mathrm{C_2}-\mathrm{C_3}$ bond is:
(a) $s p-s p^{2}$
(b) $s p-s p^{3}$
(c) $s p^{2}-s p^{3}$
(d) $s p^{3}-s p^{3}$
Show Answer
Answer
$\stackrel{6}C_2=\stackrel{5}{C} H-\stackrel{4}C_2-\stackrel{3}C_2-\stackrel{2}{C} \equiv \stackrel{1}{C} H$
In the given organic compound, the carbon atoms numbered as 1, 2, 3, 4, 5, and 6 are $s p, s p, s p^{3}, s p^{3}, s p^{2}$, and $s p^{2}$ hybridized respectively. Thus, the pair of hybridized orbitals involved in the formation of $C_2-C_3$ bond is $s p \hat{a} \epsilon^{\prime \prime} s p^{3}$.
12.37 In the Lassaigne’s test for nitrogen in an organic compound, the Prussian blue colour is obtained due to the formation of:
(a) $\mathrm{Na_4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]$
(b) $\mathrm{Fe_4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]_3$
(c) $\mathrm{Fe_2}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]$
(d) $\mathrm{Fe_3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]_4$
Show Answer
Answer
In the Lassaigne’s test for nitrogen in an organic compound, the sodium fusion extract is boiled with iron (II) sulphate and then acidified with sulphuric acid. In the process, sodium cyanide first reacts with iron (II) sulphate and forms sodium hexacyanoferrate (II). Then, on heating with sulphuric acid, some iron (II) gets oxidised to form iron (III) hexacyanoferrate (II), which is Prussian blue in colour. The chemical equations involved in the reaction can be represented as
$
\begin{matrix}
6 CN^{-}+Fe^{2+} \longrightarrow[Fe(CN)_6]^{4-} \\
3[Fe(CN)_6]^{4-}+4 Fe^{3+} \xrightarrow{x H_2 O} Fe_4[Fe(CN)_6]_3 x H_2 O \\
\text{ Prussian blue }
\end{matrix}
$
Hence, the Prussian blue colour is due to the formation of $Fe_4[Fe(CN)_6]_3$.
12.38 Which of the following carbocation is most stable?
(a) $\left(\mathrm{CH_3}\right)_3 \mathrm{C} \cdot \stackrel{+}{\mathrm{C}} \mathrm{H_2}$
(b) $\left(\mathrm{CH_3}\right)_3 \stackrel{+}{\mathrm{C}}$
(c) $\mathrm{CH_3} \mathrm{CH_2} \stackrel{+}{\mathrm{C}} \mathrm{H_2}$
(d) $\mathrm{CH_3} \stackrel{+}{\mathrm{C}} \mathrm{H} \mathrm{CH_2} \mathrm{CH_3}$
Show Answer
Answer
$(CH_3)_3 \stackrel{+}{C}$
is a tertiary carbocation. A tertiary carbocation is the most stable carbocation due to the electron releasing effect of three methyl groups. An increased + I effect by three methyl groups stabilizes the positive charge on the carbocation.
12.39 The best and latest technique for isolation, purification and separation of organic compounds is:
(a) Crystallisation
(b) Distillation
(c) Sublimation
(d) Chromatography
Show Answer
Answer
Chromatography is the most useful and the latest technique of separation and purification of organic compounds. It was first used to separate a mixture of coloured substances.
12.40 The reaction: $\mathrm{CH_3} \mathrm{CH_2} \mathrm{I}+\mathrm{KOH}(\mathrm{aq}) \rightarrow \mathrm{CH_3} \mathrm{CH_2} \mathrm{OH}+\mathrm{KI}$ is classified as :
(a) electrophilic substitution
(b) nucleophilic substitution
(c) elimination
(d) addition
Show Answer
Answer
$CH_3 CH_2 I+KOH _{(a q)} \longrightarrow CH_3 CH_2 OH+KI$
It is an example of nucleophilic substitution reaction. The hydroxyl group of $KOH(OH^{\text{af }})$ with a lone pair of itself acts as a nucleophile and substitutes iodide ion in $CH_3 CH_2$ to form ethanol.
